Our SVP of Engineering and Manufacturing brings over 30 years of experience in the electronics contract manufacturing industry. They have been a pioneer in quick-turn PCB (Printed Circuit Board) prototyping and New Product Introduction (NPI), previously leading global NPI operations at a top tier EMS vendor. Their expertise ensures the seamless integration of new products into manufacturing, a key factor in accelerating time-to-market for innovative electronic products.
Thanks to the wealth of best practices they have developed, we are recognized as leaders in quick-turn PCB prototyping and NPI. Our accuracy and efficiency have earned us a stellar reputation among engineers in Silicon Valley, the global hub of technological innovation.
Contact Us today for a free consultation on how we can help bring your products to market faster.
We do DFM (Design for Manufacturing) into the early stages of the process to Prevent the need to rework elements later in the process. We make them fit with manufacturing realities, minimize costs because issues can be dealt with early without double-work.
Our engineering support for pre-design for manufacturability (DFM) services ensures that design issues are addressed early, reducing the need for costly revisions during prototype builds. We also provide post-DFM services to minimize manufacturability challenges in future production.Read more ▶
We define manufacturing processes, including equipment and machinery requirements, establish quality control parameters, and develop detailed process documentation to ensure consistent, high-quality production.

- The Importance of Materials in Medical Devices: Materials play a critical role in the success of medical devices, often accounting for over 70% of the total product cost. A strong supply chain with stringent vendor quality control is essential to maintain profitability and competitiveness.
- Strategic Sourcing and Cost Optimization: We manage the medical device supply chain by sourcing high-quality materials from approved suppliers. Our Far East procurement team ensures cost optimization, while our component engineers identify alternative parts to address lead-time challenges and reduce material costs, helping clients manage expenses efficiently.
- Leveraging Advanced ERP Systems for Supply Chain Management: SAP ERP System allows us to effectively manage supply and demand, ensuring the highest material quality and availability. This ensures smooth operations and timely deliveries, making us a reliable partner in medical device manufacturing.
Manufacturing Process:
We offer a wide range of medical device assembly services, including:
- Printed Circuit Board Assemblies (PCBAs): Using surface mount technology (SMT) or through-hole technology (THT), we solder components onto PCBs.Read more ▶
- Sheet Metal Fabrication and Welding: To cut, bend, and shape thin metal sheets to create various components.
- Injection Molding of Plastic Parts: To produce precise, repeatable plastic components through injection molding.
- Man-Machine Interface (MMI): To design and integrate user interfaces, such as buttons, LED indicators, and touchscreens, for medical devices.
Medical Device Quick-Turn Prototyping:
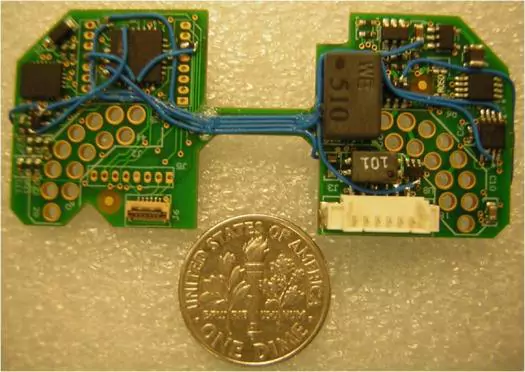
- Importance of Quick-Turn PCB Assembly for Prototyping: Quick-turn PCB assembly (PCBA) prototypes play a crucial role in expediting product development and reducing time-to-market. We offer DFM/DFT engineering services to ensure the prototype is done right the first time. Our quickest turn for PCB assembly prototypes is within 24 hours.Read more ▶
- Skilled Engineering Support for Prototype Reworking: Our engineers and technicians are very skilled in reworking prototypes to avoid the respinning of the PCB design; it saves cost and time to prove the prototype design. This is extremely crucial when there is a tight project schedule.

- Success Story of Rapid Prototype Reworking: There was a case where the customer didn't have time to respin the prototype PCB. We helped to add 26 jumper wires to both sides of an extremely small board to make the prototype functional. It helped the customer to show the new product at the trade show with great success.
- Success Story of adding wire from "under BGA" to a circuit: This task is used to change a circuit path at a BGA site for engineering changes/modifications. This process requires precision equipment and highly trained technicians.
New Product Introduction (NPI):
New Product Introduction (NPI) is a critical process that bridges the gap between product development and mass production of medical equipments. We have a a well-defined NPI process to identify potential issues earlier, streamlining process for mass production, cost optimization, and quality assurance.Read more ▶
Devices Verification
Verification is a crucial process in the development and manufacturing of medical devices, ensuring that the device is built correctly according to design specifications. So that the devices are safe, effective, and meet regulatory standards.
Testing: We utilize state-of-the-art testing equipment to perform rigorous functional and performance testing on every device we manufacture. This ensures each product functions flawlessly before reaching patients.
Read more ▶
Packaging and Labeling:
We apply clear, accurate product labels and package products according to regulatory requirements and customer specifications.
Read more ▶
Medical device Quality Assurance (QA):

Regulatory Compliance: We ensure that all processes adhere to ethical standards and comply with relevant medical device regulations (e.g., FDA, ISO 13485, UL, CE) and regularly update our compliance procedures in meeting the highest safety and quality requirements for patient well-being.Read more ▶
Structured Methodology: Our quality control methodology is designed to ensure the highest levels of safety, effectiveness, and reliability for medical devices. This includes thorough material inspections, controlled manufacturing processes, and comprehensive testing, which are crucial for medical device assembly and manufacturing.
Traceability and Transparency: We maintain a comprehensive traceability system that allows us to track every component and material used in each device. This ensures we can quickly identify and address any potential quality issues. We dedicated to manufacture medical equipment meeting the quality standards and preventing defects and recalls while safeguarding patient safety. We maintaimaintains medical device history records (DHR) in accordance with the requirements of the FDA regulations. Our Quality System ensures that DHR's are maintained in accordance with the Device Master Record (DMR).

Computerized Manufacturing Execution System (MES), coupled with real-time barcode data collection, to track the movement of components and products throughout the manufacturing process. It enhances quality control and ensures traceability and regulatory compliance per medical regulations, such as unique device identification (UDI), etc. We customize our traceability system to meet specific customer needs for medical device and equipment manufacturing.
Continuous improvement: "We are committed to continuous improvement in our quality control processes. We regularly evaluate and update our procedures to ensure we remain at the forefront of medical device safety"
Regular Quality Review Meeting:
We conduct regular quality meetings with our OEM customers to ensure we consistently meet their requirements and enhance their satisfaction. During these meetings, we review the mutually agreed-upon KPIs to drive continuous improvement.
Distribution of Medical Equipment:

We coordinate with logistics providers for efficient product delivery to the designated locations, manage inventory, and optimize distribution channels to reduce costs and speed up time-to-market for medical equipment manufacturing.
After-sales Support:
It is a critical aspect of the services we provide to our customers, including repair, warranty services, technical support with troubleshooting, spare parts management, handling returns, repairs, and refurbishments, etc.
Sustainability:
We implement environmentally friendly practices, such as waste reduction and energy conservation, and source materials from sustainable suppliers when possible.
Option for Offshore Medical Device Manufacturing:
We have adequate capacity at our Fremont, CA assembly facility, and our Taiwan assembly facility offers an excellent option for high-volume products. We provide competitive offshore manufacturing services at our Taiwan facility, enhancing our clients' competitiveness and helping them capture a larger market share. Taiwan boasts exceptional supply chains for electronic products, further supporting our high-quality manufacturing capabilities.Read more ▶
🤝 Customer Support and Collaboration
We assign dedicated Customer Focus Team (CFT) to assist you at every stage of your project. From initial consultation to final delivery. Our CFT team works closely with you to proactively understand your unique requirements, help you well-plan your projects early, and provide tailored solutions. Our collaborative approach ensures seamless communication and successful project outcomes.Read more ▶
We have a culture of continuous improvement and are always open to your feedback and suggestions. Your opinions are very important to us.
🤝 Your Trusted Partner in
Medical Equipment Manufacturing
Developing a lasting partnership in medical device manufacturing goes beyond typical manufacturing relationships. It's built on shared goals, values, and a long-term vision for success. As your strategic partner, we invest in your success by providing comprehensive support that extends beyond production.
Our unwavering commitment to excellence in precision medical equipment assembly has established us as a preferred vendor in the industry. We pride ourselves on delivering:
- Unmatched quality
- Rapid turnaround
- Responsive service
- Consistent results
With our proven track record, we have confidence in meeting your manufacturing needs with precision and efficiency. Count on us as your trusted partner for all your medical device manufacturing requirements.
















